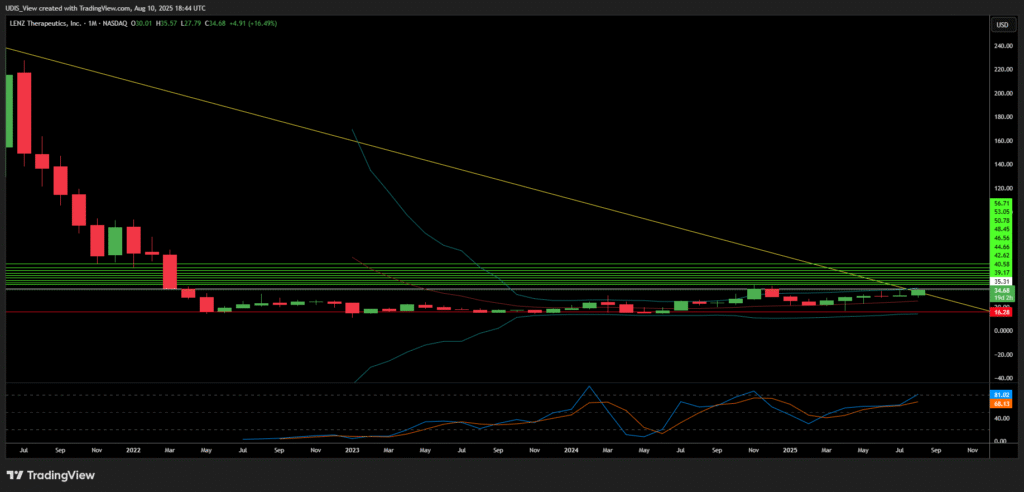
LENZ Therapeutics, Inc. (NASDAQ: LENZ) dominates the presbyopia treatment landscape with its groundbreaking VIZZ eye drops. The FDA approved VIZZ on July 31, 2025, marking a pivotal milestone. This aceclidine-based solution restores near vision for up to 10 hours daily without glasses. It targets 128 million U.S. adults and 1.8 billion globally affected by presbyopia. Stock surged 7.97% to $34.68 by August 8, 2025, hitting a 52-week high of $38.93. Analysts rate it a strong buy, with price targets up to $56. LENZ expands internationally through partnerships in China and Canada. This article dissects growth reasons across geopolitics, geostrategy, macroeconomics, economics, technology, cyber, science, high-tech, and patents.
Geopolitics
LENZ leverages U.S.-China healthcare ties despite broader tensions. CORXEL Pharmaceuticals submitted an NDA for LNZ100 (VIZZ’s precursor) to China’s NMPA, triggering a milestone payment. This taps China’s aging population amid U.S.-China trade strains. LENZ secures up to $95 million in milestones and double-digit royalties. In Canada, a license with Laboratoires Théa yields over $70 million in payments plus royalties. These deals counter geopolitical risks like tariffs, ensuring revenue streams. U.S.-Canada alliances stabilize supply chains. LENZ’s moves affirm resilience in fragmented global politics.
Geostrategy
LENZ positions itself as a global leader in ophthalmic innovation. Licensing LNZ100 to CORXEL in Greater China accesses a massive market, bypassing direct exposure to U.S.-China conflicts. The Canadian deal with Théa enhances North American dominance. These strategic alliances diversify risks and accelerate commercialization. LENZ avoids heavy manufacturing in volatile regions, focusing on U.S.-based operations. Positive Phase 3 data from China reinforces global efficacy. This geostrategic footprint drives valuation growth, with stock forecasts reaching $52.40 average in 2025.
Macroeconomics
Aging demographics fuel LENZ’s ascent. Global healthcare spending rises with populations over 45, where presbyopia prevalence spikes. U.S. inflation moderates, boosting biotech investments. Low interest rates favor growth stocks like LENZ. Post-pandemic recovery amplifies demand for non-invasive treatments. LENZ benefits from stable currencies in key markets. Economic expansion in China and Canada supports partnerships. Stock climbed to $34.68 amid bullish macro trends, with analysts predicting 40% upside.
Economics
Presbyopia market economics propel LENZ forward. The global market hits $20.17 billion in 2025, growing to $29.54 billion by 2032 at 5.6% CAGR. VIZZ’s once-daily dosing captures share from glasses and competitors like Vuity. LENZ prices VIZZ at $79 monthly, targeting high margins. Partnerships yield $165 million in milestones and royalties. Reduced side effects drive adoption, outpacing pilocarpine’s issues. Economic viability shines with no serious adverse events in trials. Stock targets soar to $87 high.
Technology
VIZZ’s aceclidine technology revolutionizes presbyopia care. It selectively contracts the iris sphincter, creating a pinhole effect for sharp near vision. Unlike pilocarpine, it spares ciliary muscles, avoiding headaches or retinal risks. Preservative-free, single-use vials ensure sterility. Onset hits in 30 minutes, lasting 10 hours. Phase 3 trials confirm 71% three-line improvement at three hours. LENZ integrates advanced formulation tech for stability. This tech edge boosts stock performance.
Cyber
LENZ fortifies against pharma’s cyber vulnerabilities. Industry faces ransomware and IP theft, but LENZ employs cookies for site security and analytics. No breaches reported, unlike peers. Robust protocols protect trial data and patents. Employee training mitigates phishing. Multi-layered defenses include firewalls and encryption. Cyber resilience safeguards expansion, sustaining investor confidence. Stock resilience post-approval underscores strong defenses.
Science
Aceclidine’s science underpins LENZ’s success. It acts as a muscarinic M3 agonist, shrinking pupils without myopic shift. This pinhole mechanism enhances depth of focus. Trials show no serious events over 30,000 days. VIZZ outperforms Vuity by minimizing brow ache. Scientific validation from CLARITY studies drives adoption. Peer reviews pending, but FDA nod affirms efficacy. LENZ leads in pupil-selective miotics.
High-Tech
LENZ harnesses high-tech in ophthalmic delivery. VIZZ’s 1.44% aceclidine solution uses advanced engineering for quick onset and duration. Single-dose vials leverage precision manufacturing. Digital tools track partnerships and trials. High-tech formulation avoids preservatives, reducing irritation. Integration with e-pharmacies speeds access by Q4 2025. This innovation elevates LENZ above competitors, fueling stock gains.
Patent Analysis
Patents solidify LENZ’s moat. U.S. patents cover aceclidine derivatives, compositions, and presbyopia methods. FI3542798T3 and ES3028364T3 protect formulations at 1.25-1.85% concentrations. Storage-stable aceclidine patents ensure exclusivity. These barriers deter generics, extending market dominance. Patent portfolio supports global filings, aligning with China and Canada deals. Strong IP drives valuation, with forecasts to $87.
References:
- Effects of geopolitical strain on global pharmaceutical supply chain design and drug shortages
- The Contentious U.S.-China Trade Relationship
- CNA Statement on Canada-U.S. Geopolitical Tensions and Health Care
- STOCKSCAN
- North American Trade War? The Geopolitical Impacts for China and the United States
- Global Presbyopia Market – Industry Trends and Forecast to 2029
- Stockinvest.us
- Presbyopia Global Market Report 2025
- Presbyopia Treatment Market SIZE AND SHARE ANALYSIS – GROWTH TRENDS AND FORECASTS (2025-2032)
- Stock Spotlight: LENZ Therapeutics (NASDAQ: LENZ)
- FDA approves Lenz Therapeutics’ VIZZ for the treatment of presbyopia
- Vizz Becomes First, Only FDA-Approved Aceclidine-Based Eye Drop for Presbyopia
- FDA Approval Alert
- lenz-tx.com
- Cybersecurity Risks and Solutions for the Pharmaceutical Industry
- FDA Approves Aceclidine (VIZZ) for Presbyopia in Adults
- An updated systematic review of pharmacological treatments for presbyopia
- HIGHLIGHTS OF PRESCRIBING INFORMATION
- Aceclidine for use in the treatment of presbyopia – ES3028364T3 – Spain
- Aceclidine for use in the treatment of presbyopia – FI3542798T3 – Finland
- Patents Assigned to LENZ THERAPEUTICS OPERATIONS, INC.
LENZ Therapeutics Long (Buy)
Enter At: 35.31
T.P_1: 39.17
T.P_2: 40.58
T.P_3: 42.62
T.P_4: 44.66
T.P_5: 46.56
T.P_6: 48.45
T.P_7: 50.78
T.P_8: 53.05
T.P_9: 56.71
S.L: 16.28